
This poster was originally presented at the Professional Society for Health Economics and Outcomes Research (ISPOR) Europe 2024 Annual Meeting, held November 17-20, in Barcelona, Spain.
Authors: Karen E. Smoyer, Dave Iwanyckyj, Pablo Racana, and Suki Kandola
Affiliations: Amplity, Langhorne, PA, USA; Envision Pharma Group, Fairfield, CT, USA; and Envision Pharma Group, London, UK
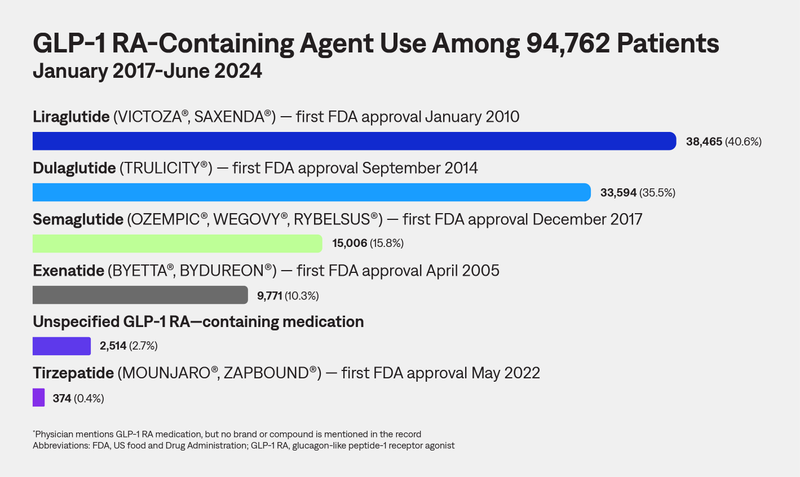
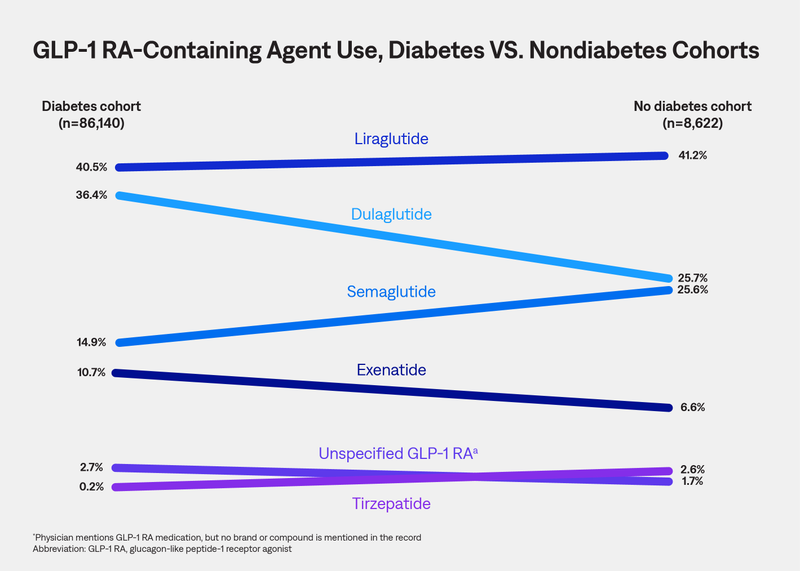
This study leveraged AnswerY™ (formerly known as Amplity Insights), our robust real-world database (RWD), including HIPAA-compliant physician notes, to characterize real-world use of GLP-1 RA–containing medications among adults in the United States.
Turn real insights into real impact with our AI-powered platform.