
No image found.
This article was originally written for and published in Journal of Oncology Navigation & Survivorship (JONS). Visit JONS for more wholistic discussions about evidence-based practices in navigating patients with cancer and their caregivers.
Gastric cancer ranks among the most prevalent cancers globally. A significant number of patients receive their diagnosis at advanced stages, primarily because the early symptoms are often subtle, and regular screening is infrequently conducted.1
Systemic chemotherapy is the mainstay treatment for metastatic gastric cancer1; however, the use of targeted therapies has evolved significantly. Some examples of these therapies include trastuzumab, a monoclonal antibody that targets HER2; ramucirumab, a monoclonal antibody that targets the VEGFR2 protein; and zolbetuximab, a monoclonal antibody that targets CLDN18.2.
Ramucirumab was approved in April 2014 for the treatment of advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma with disease progression on or after prior fluoropyrimidine- or platinum-containing chemotherapy.
Ten years later, in October 2024, zolbetuximab was approved for patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumors are CLDN18.2 positive.3
Trastuzumab deruxtecan was approved for HER2- positive gastric or GEJ adenocarcinoma in January 2021 for patients who have received a prior trastuzumab-based regimen.4
Even with the approved targeted therapies for gastric cancer, many patients are still not receiving targeted therapy.
Real-world data can be used to further understand trends in biomarker testing and the use of targeted therapies among patients with gastric cancer. AnswerY™ is a unique database and platform that features transcribed physician notes from the United States. Utilizing natural language processing, it extracts structured information from comprehensive patient visit records. With more than 80 million entries, this database enables users to derive actionable insights from real-world data that extend beyond mere claims and ICD-10 codes. AnswerY was searched for patients diagnosed with gastric cancer (from 2017 to 2025; grouped as before 2021 and after 2021) for any mention of “biomarker,” “biomarker tested,” and “biomarker positive.” It was also used to search for mentions of patients who were biomarker positive and prescribed any line of targeted therapy.
AnswerY is a unique database that features transcribed physician notes from the United States. Utilizing natural language processing, it extracts structured information from comprehensive patient visit records.
Before 2021, 76% of patients with gastric cancer were tested for biomarkers; after 2021, 69% of patients with gastric cancer were tested for biomarkers. HER2 (<2021, 69%; 2021+, 65%) and PD-L1 (<2021, 25%; 2021+, 28%) biomarkers were found most frequently. The Figure shows the percentage of patients who were biomarker positive and received targeted therapy before and after 2021. Mentions for targeted therapy were found for 4 biomarkers: HER2, PD-L1, TMB, and BRAF. The number of patients who were biomarker positive and on a targeted therapy was low—between 30% and 38% before 2021 and 16% and 59% after 2021—with the majority of patients on targeted therapy for HER2- and PD-L1–positive disease.
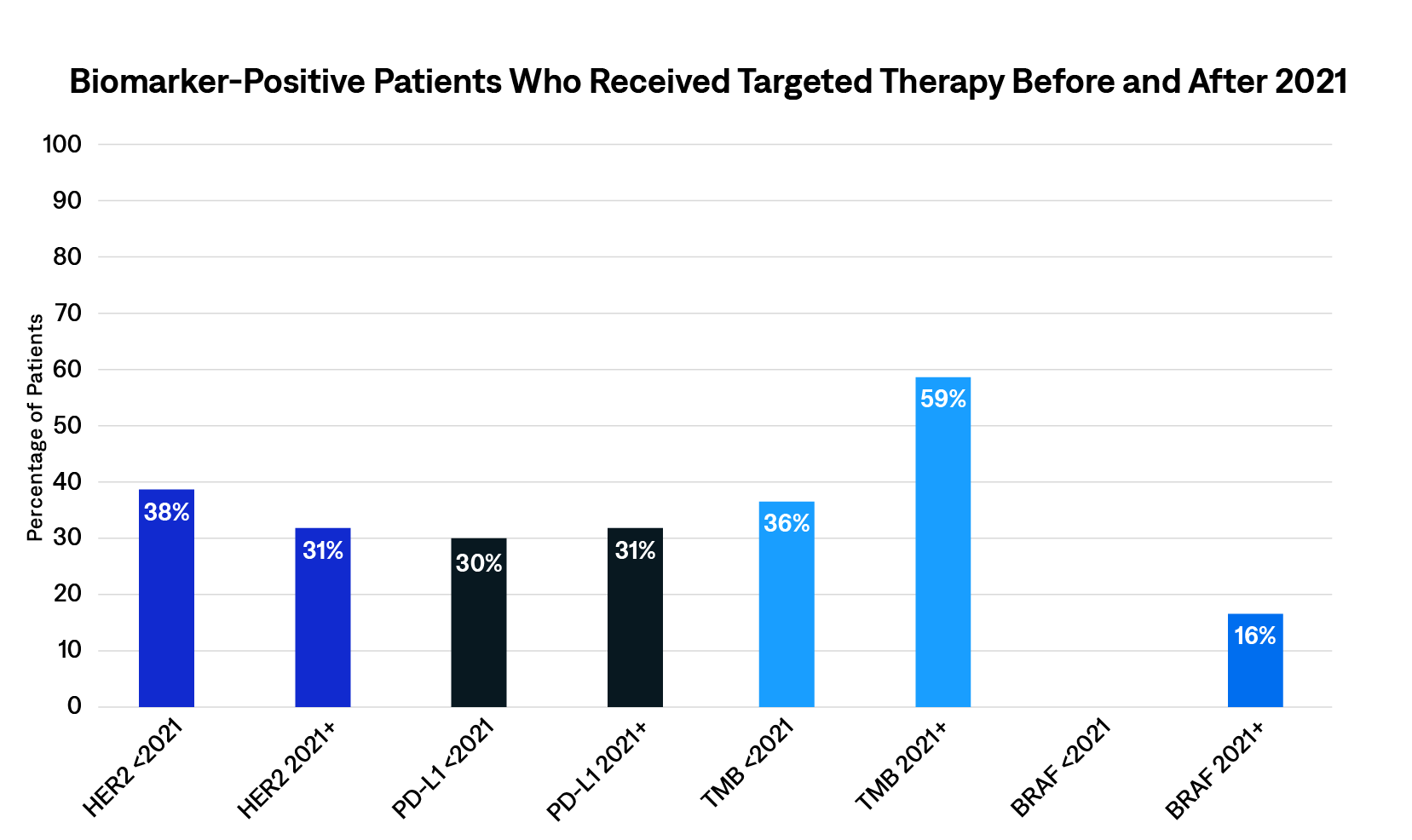
This real-world analysis showed that not many patients who are biomarker positive are receiving targeted therapy. The low number of patients who were biomarker positive and on a targeted therapy may be due to multiple factors. One factor may be that chemotherapy is generally used in the first line of therapy, while targeted therapy is usually indicated in the second line of therapy,5,6 with the exception of zolbetuximab.7 In addition, the use of targeted therapies is restricted to a specific subset of the population with gastric cancer due to a small fraction of patients presenting with specific biomarkers.8
The low amount of targeted therapy being used in biomarker-positive patients may also be due to sociodemographic or socioeconomic factors. A study on sociodemographic disparities in targeted therapy for ovarian cancer included patients with ovarian cancer (stage I-IV) between 2012 and 2019 from the National Cancer Database.9
Among the 99,286 patients with ovarian cancer, only 4.1% received targeted therapy. Variations were observed in the administration of targeted therapies based on the age at which the diagnosis was made, the stage of the disease, and the presence of comorbidities at the time of diagnosis, as well as factors influencing healthcare access, including health insurance status.9
This analysis conducted in a real-world setting revealed that a limited number of biomarker-positive patients are being administered targeted therapy.
This is consistent with what is seen in the literature and may be due to limited targeted therapies being used in the first-line setting as well as socioeconomic and sociodemographic factors.
More research is needed to make targeted therapies available to patients with gastric cancer.
Zoom In: Explore other biomarker research based on AnswerY, our AI platform of HIPAA-compliant patient-provider conversations.