
This poster was originally presented at ISPOR 2025 on May 15, in Montreal, QC, Canada.
Authors: Abbey Nakano, PharmD; Dave Iwanyckyj, BA; Fernando Otalora; John Perez; Melanie Jardim, PhD
Affiliations: Amplity, Inc., Langhorne, PA, USA
This study aims to assess real-world prescribing patterns of biologics and explore factors driving therapy change for patients with ulcerative colitis (UC) and Crohn’s disease (CD).
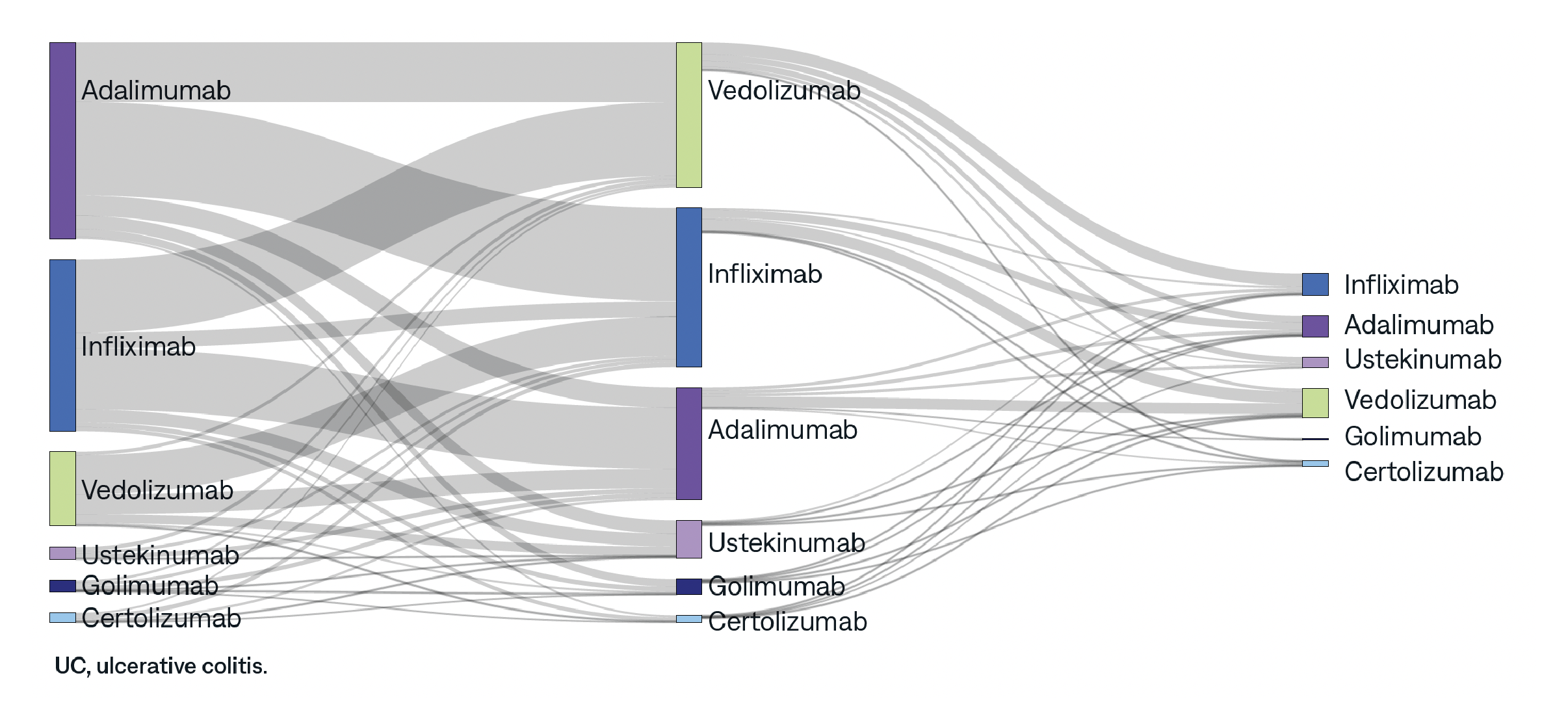
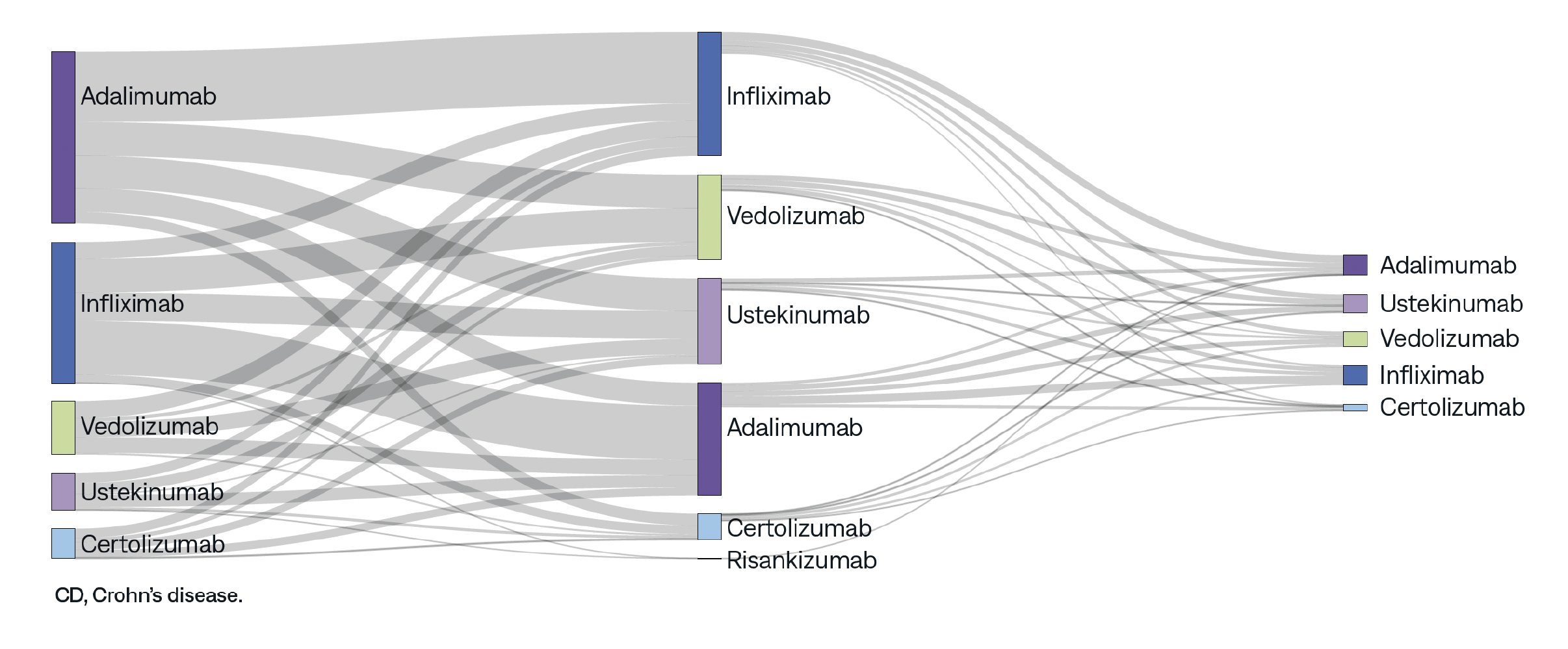
AnswerY helps uncover hidden insights. Explore other research topics, including disparities in healthcare access, Alzheimer’s Disease, and biomarker testing in NSCLC.